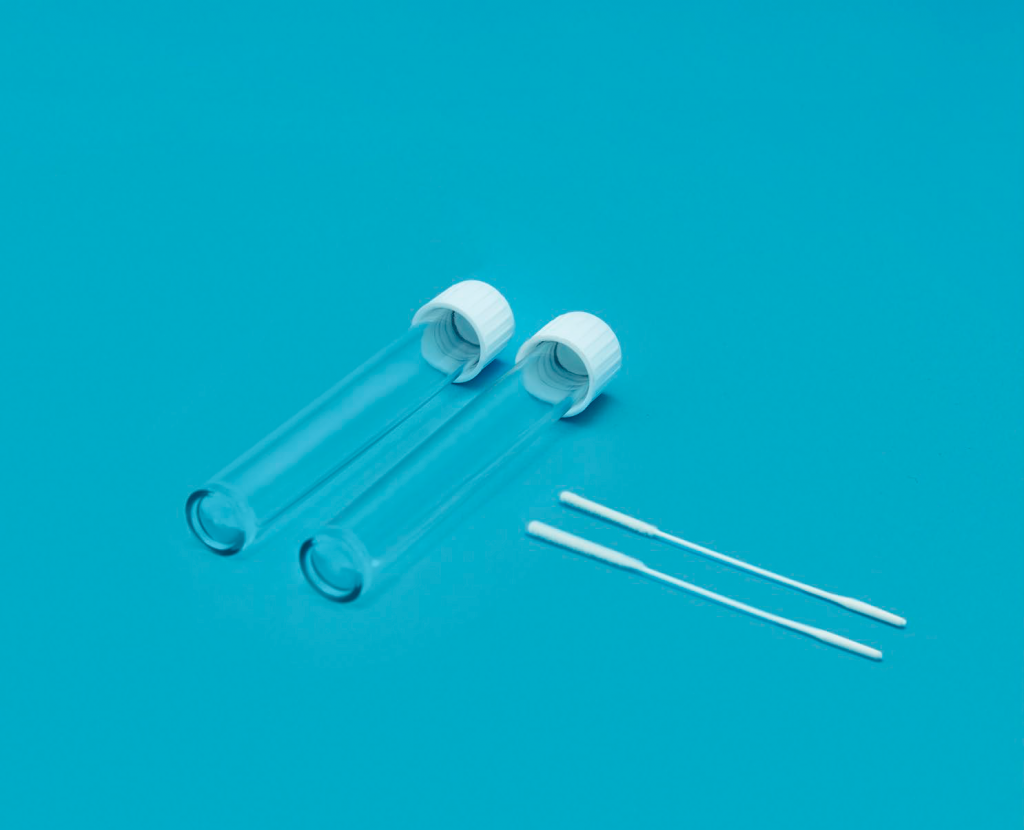
DiagnoVTM v2.0
DiagnoVTM v2.0 are designed to collect clinical samples containing virus from the area where secretions from the nasal cavity (nasopharynx) and throat (oropharynx) are collected and transported to the test laboratory. Samples collected with swabs are stored in this viral transport medium. It can be used for virus testing and isolation in the laboratory with the Polymerase Chain Reaction (PCR) method.
Diagno VTM v2.0 is certified and marked with CE-IVD.

MAGICPREP KIT 2
Magicprep Tubes contains 3mL of viral nucleic acid extractive and preservative liquid. When clinical specimens suspected of respiratory tract infection are transferred in this tube, the liquid inside the tube can be used directly in real-time PCR (qPCR) reactions. The nucleic acid extractive and protective liquid inactivates all viral, bacterial or eukaryotic pathogens in the sample 5 minutes after contact with the clinical specimen.
MAGICPREP KIT 2 is certified and marked with CE-IVD.
DiagnoSaliva Extraction Kit
DiagnoSaliva Extraction Kit is designed for simple and non-invasive saliva collection and preservation of DNA/ RNA in saliva samples at ambient temperature. Saliva samples are collected by spitting inside the Collection Tube. After collecting 1-1.5 mL of saliva then saliva specimens are processed with the DiagnoSaliva Processing Buffer and finally ready to use for RT-PCR reactions. This kit is ideal for collecting and preserving saliva samples for detecting Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) by DIAGNOVITAL HS SARS-CoV-2 Real-Time PCR Kit v2.0.
DiagnoSaliva Extraction Kit is certified and marked with CE-IVD.
