
Rapidfor® Antigen Rapid Test Kit (Professional Use)(Nasal)
SARS-CoV-2 Antigen Rapid Test Kit is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasopharyngeal swab specimens from individuals who meet Covid-19 clinical criteria.
SARS-CoV-2 Antigen Rapid Test Kit detects current infection during the acute phase of Covid-19, while the coronavirus is still present in large quantities in the respitory tract (nasopharynx).
RapidFor ™ Covid-19 Antigen Rapid Test Kit is for professional and home use is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
Rapidfor® Antigen Rapid Test Kit is certified and marked with CE-IVD.

Rapidfor® Saliva Antigen Rapid Test Kit
SARS-CoV-2 Saliva Antigen Rapid Test Kit is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 Antigen (Ag) in human saliva specimens from individuals who meet Covid-19 clinical criteria.
SARS-CoV-2 Saliva Antigen Rapid Test Kit detects current infection during the acute phase of Covid-19, while the coronavirus is still present in large quantities in the respitory tract (nasopharynx).
RapidFor ™ Covid-19 Saliva Antigen Rapid Test Kit is for home use and is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
Rapidfor® Saliva Antigen Rapid Test Kit is certified and marked with CE-IVD.

RapidFor® SARS-CoV-2 Antigen Test Kit (Self Test)
SARS-CoV-2 Antigen Rapid Test Kit is an in vitro diagnostic rapid test for the qualitative detection of SARS-CoV-2 antigen (Ag) in human nasopharyngeal swab specimens from individuals who meet Covid-19 clinical criteria.
SARS-CoV-2 Antigen Rapid Test Kit detects current infection during the acute phase of Covid-19, while the coronavirus is still present in large quantities in the respitory tract (nasopharynx).
RapidFor ™ Covid-19 Antigen Rapid Test Kit is for home use is intended to be used as an aid in the diagnosis of SARS-CoV-2 infection.
RapidForTM SARS-CoV-2 Antigen Test Kit is certified and marked with CE-IVD.

Cirrus COVID-19 Antigen Test
Cirrus COVID-19 Antigen Test is intended to be used for qualitative detection of SARS-CoV-2 nucleocapsid antigen in nasopharyngeal and oropharyngeal swab specimens from patients suspected of COVID-19. The antigen can generally be detected in swab specimens taken during the acute phase of the infection. Positive results indicate that nucleocapsid antigen of SARS-CoV-2 was detected, however, it is necessary to check clinical correlation with patient’s history and other diagnostic findings to determine the infection status.
Cirrus COVID-19 Antigen Test is an in vitro diagnostic medical device (IVD) intended for professional use. The test should be used by trained clinical laboratory personnel.
Cirrus COVID-19 Antigen Test is an in vitro diagnostic medical device (IVD) intended for professional use. The test should be used by trained clinical laboratory personnel.
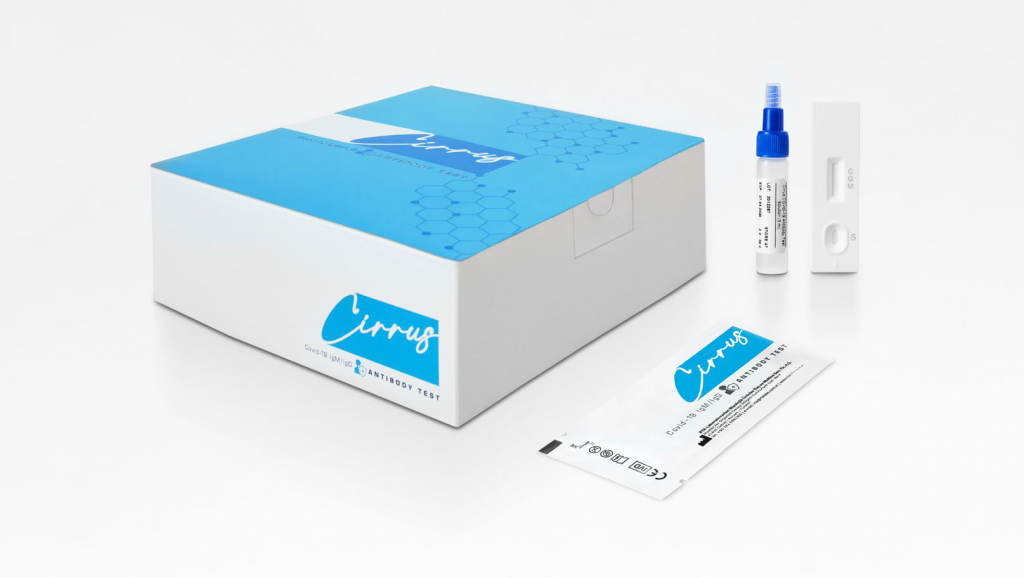
Cirrus COVID-19 IgM/IgG Antibody Test
Cirrus COVID-19 IgM/IgG Antibody Test is a rapid and qualitative immunochromatographic in vitro assay for differential detection of IgM and IgG antibodies to SARS-CoV-2 in human serum, plasma and whole blood samples. The device is designed to aid in the determination of recent or previous exposure to SARS-CoV-2 virus, tracking the body’s immunity status after infection by the SARS-CoV-2 virus. It only provides a preliminary result. The test is in vitro diagnostic (IVD) device for professional use only.
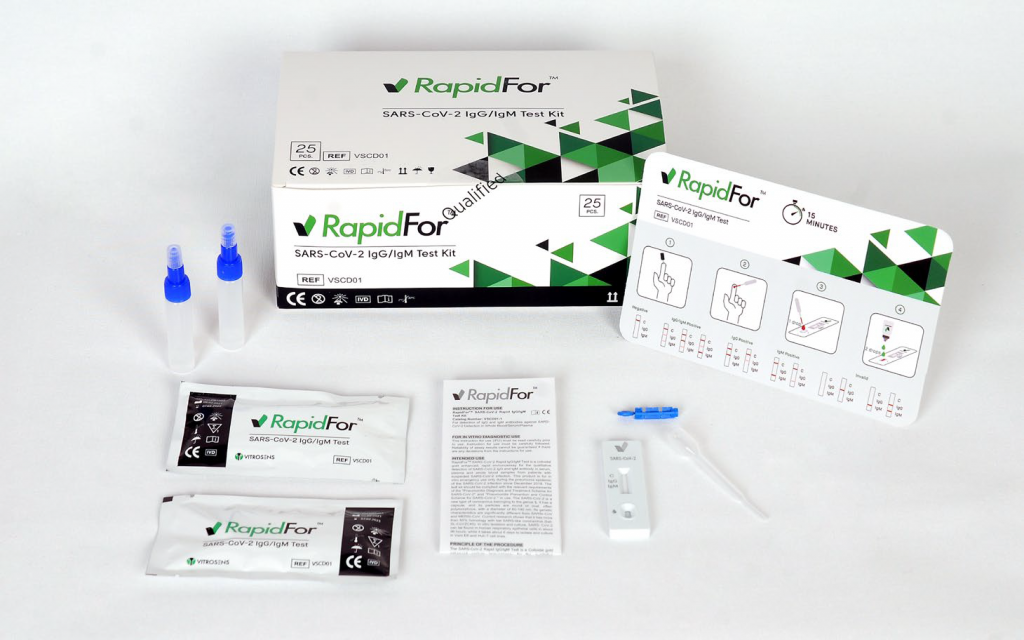
Rapidfor® IgG IgM Rapid Test Kit
RapidFor® Covid-19 IgG/IgM Rapid Test Kit is a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to SARS-CoV-2. This test kit detects the presence of IgG and IgM antibodies in serum, plasma and whole blood samples.
Antibody Rapid Test Kit detects whether you had COVID-19 in the past and now have antibodies against the virus. Antibodies are proteins made by the immune system to fight infections like SARS-CoV-2. Antibodies can take days or weeks to develop in the body following exposure to a SARS-CoV-2 (COVID-19) infection. IgM antibody is the earliest antibodies in Covid-19 infection. IgG antibodies appears later in the blood. IgG antibodies stay in your blood long after the Covid infection goes away.
Rapidfor® Saliva Antigen Rapid Test Kit is certified and marked with CE-IVD.

Celltrion DiaTrust™ COVID-19 Ag Rapid Test
Celltrion DiaTrust™ COVID-19 Ag Rapid Test is a rapid test based on lateral flow immunoassay intended for the qualitative detection of nucleocapsid and receptor binding domains (RBDs) from the SARS-CoV-2 spike proteins in human nasopharyngeal swab specimen. Results are for the identification of SARS-CoV-2 nucleocapsid and RBD protein antigen.
- NASOPHARYNGEAL SWAB SAMPLE(NPS)
- FOR USE UNDER THE EMERGENCY USE AUTHORIZATION (EUA) ONLY
- FOR IN VITRO DIAGNOSTIC USE
- FOR PRESCRIPTION USE ONLY
- FAST AND PRECISE RESULT IN 15 MINS
For use under the Emergency Use Authorization (EUA) only
